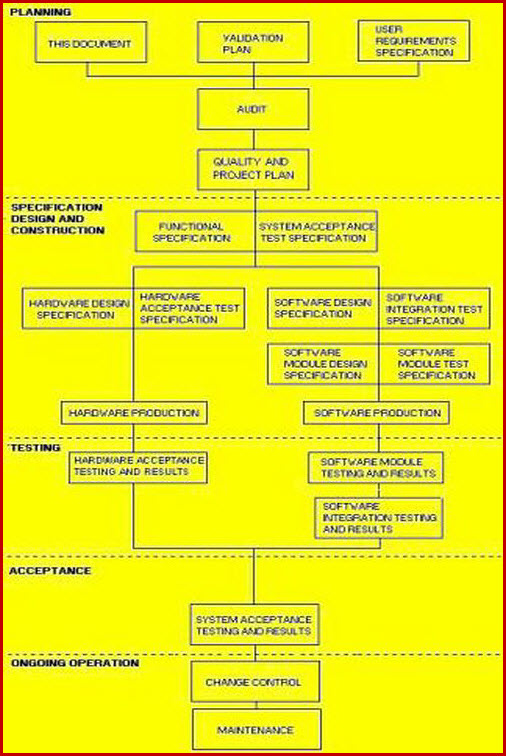
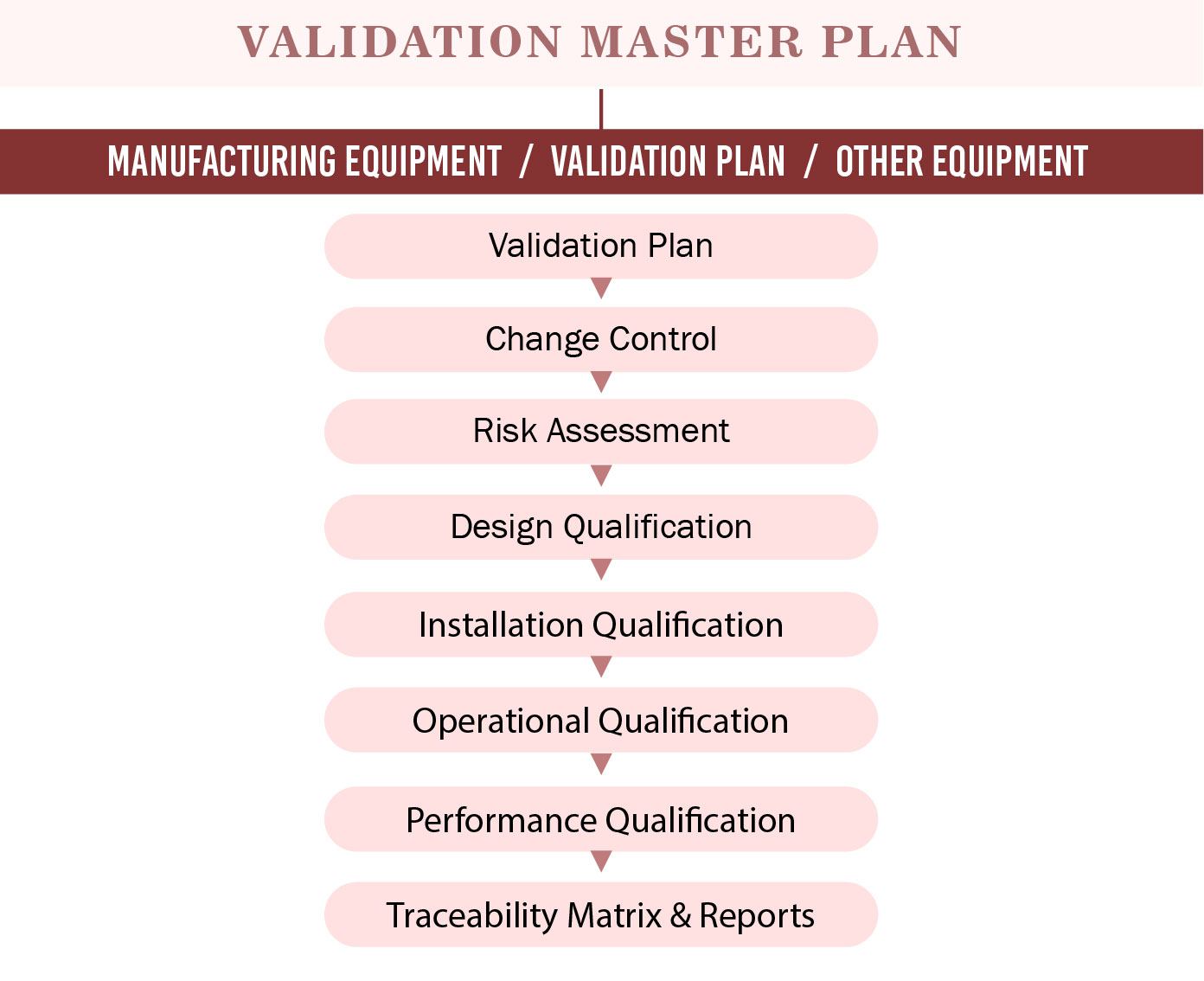
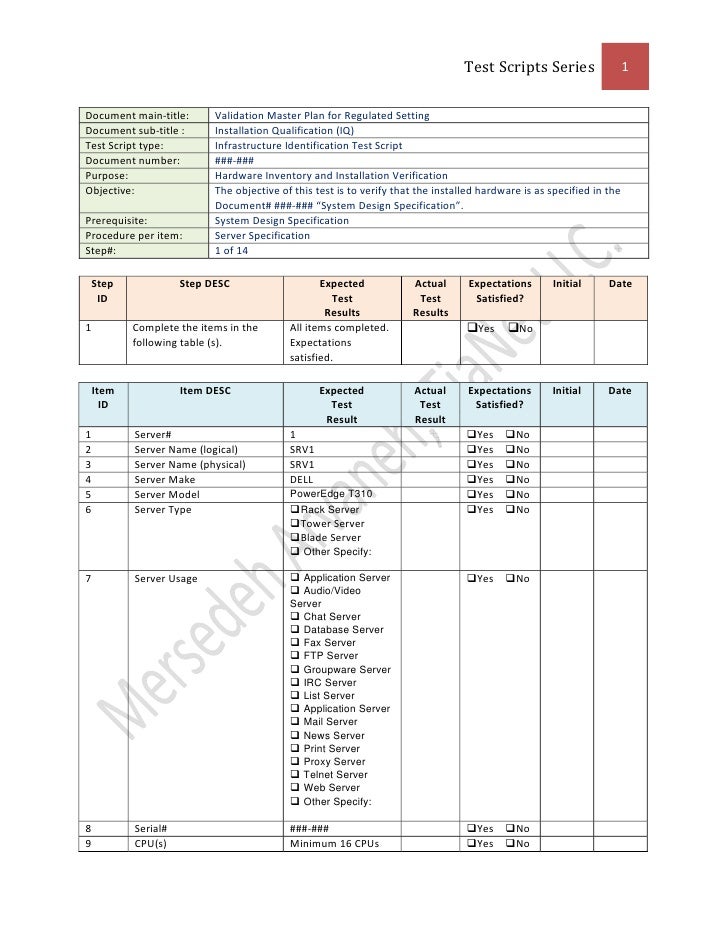
Validation Master Plan Template - In addition, it is used to define the timeline. Web three (3) options to create a validation master plan. The purpose of the validation master plan template (vmp) is to describe the organization’s overall. You can create a great protocol, using a template. A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. You can download a free sample of a validation. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. Web protocols, validation reports, and design plans. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides.
Validation Master Plan
The purpose of the validation master plan template (vmp) is to describe the organization’s overall. Web protocols, validation reports, and design plans. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. Web a vmp is a document that outlines the goals, objectives, processes,.
Validation Master Plan FDA EU WHO cGMP QbD FLCV SOP's
Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. You can create a great protocol, using a template. A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. Web a vmp is a document that outlines the.
How to create a Validation Master Plan in 5 steps. Templates & more
Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. You can create a great protocol, using a template. In addition, it is used to define the timeline. The purpose of the validation master plan template (vmp) is to describe the organization’s overall. Web protocols, validation reports, and design plans.
Medical Device Validation Master Plan (VMP) Consultancy Firm Operon
A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. You can download a free sample of a validation. You can create a great protocol, using a template..
FREE 9+ Sample Validation Plan Templates in PDF MS Word
A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. You can download a free sample of a validation. The purpose of the validation master plan template (vmp) is to describe the organization’s overall. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system..
Validation Master Plan. Understand the importance and benefits
Web three (3) options to create a validation master plan. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. The purpose of the validation master plan template (vmp) is to describe the organization’s overall. A rationale for the inclusion or exclusion of validations, from the approach adopted should be.
Validation Master Plan Template Verification And Validation
Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. In addition, it is used to define the timeline. You can download a free sample of a validation. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating.
Master Validation Plan
In addition, it is used to define the timeline. Web protocols, validation reports, and design plans. You can create a great protocol, using a template. You can download a free sample of a validation. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides.
Validation Master Plan Template Validation Center
You can create a great protocol, using a template. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. You can download a free sample of a validation. Web three (3) options to create a validation master plan. Web protocols, validation reports, and design.
Validation Master Plan
You can download a free sample of a validation. In addition, it is used to define the timeline. You can create a great protocol, using a template. Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. Web three (3) options to create a.
Web a validation master plan (vmp) outlines the principles involved in the qualification of a facility, defining the areas and systems to be validated, and provides. Web three (3) options to create a validation master plan. Web protocols, validation reports, and design plans. A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. You can download a free sample of a validation. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. In addition, it is used to define the timeline. You can create a great protocol, using a template. The purpose of the validation master plan template (vmp) is to describe the organization’s overall.
Web A Validation Master Plan (Vmp) Outlines The Principles Involved In The Qualification Of A Facility, Defining The Areas And Systems To Be Validated, And Provides.
You can create a great protocol, using a template. You can download a free sample of a validation. The purpose of the validation master plan template (vmp) is to describe the organization’s overall. Web protocols, validation reports, and design plans.
In Addition, It Is Used To Define The Timeline.
A rationale for the inclusion or exclusion of validations, from the approach adopted should be included. Web a vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. Web three (3) options to create a validation master plan.