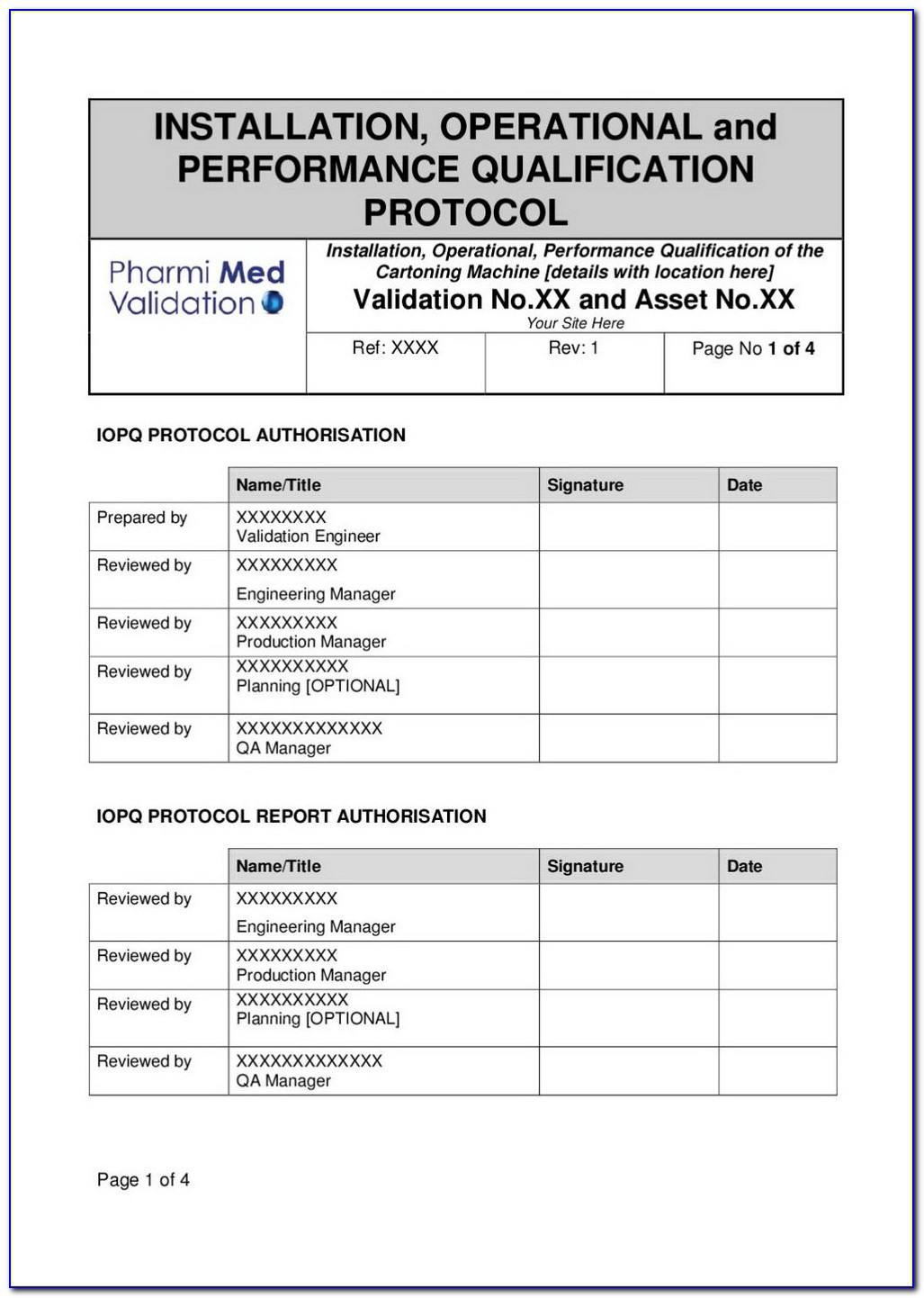
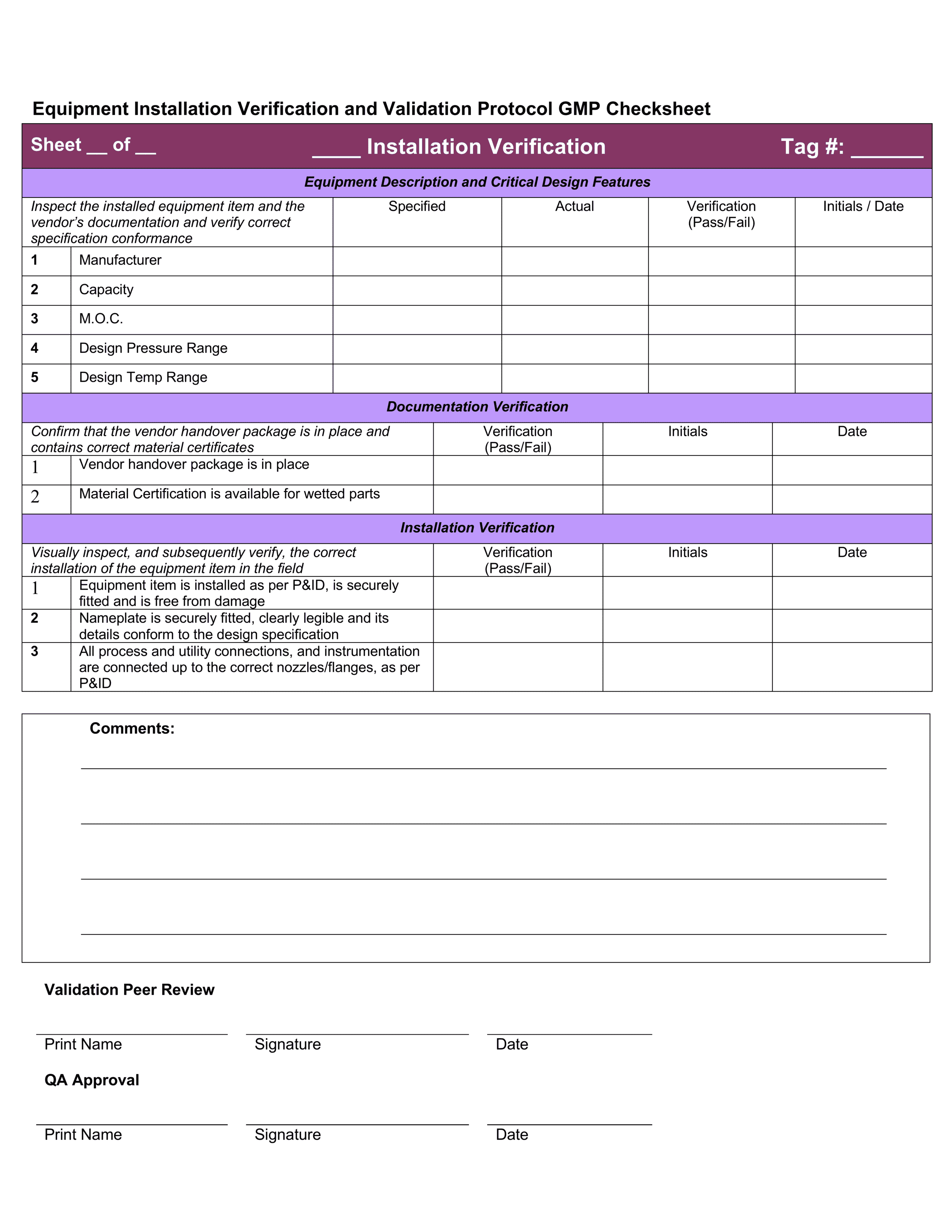
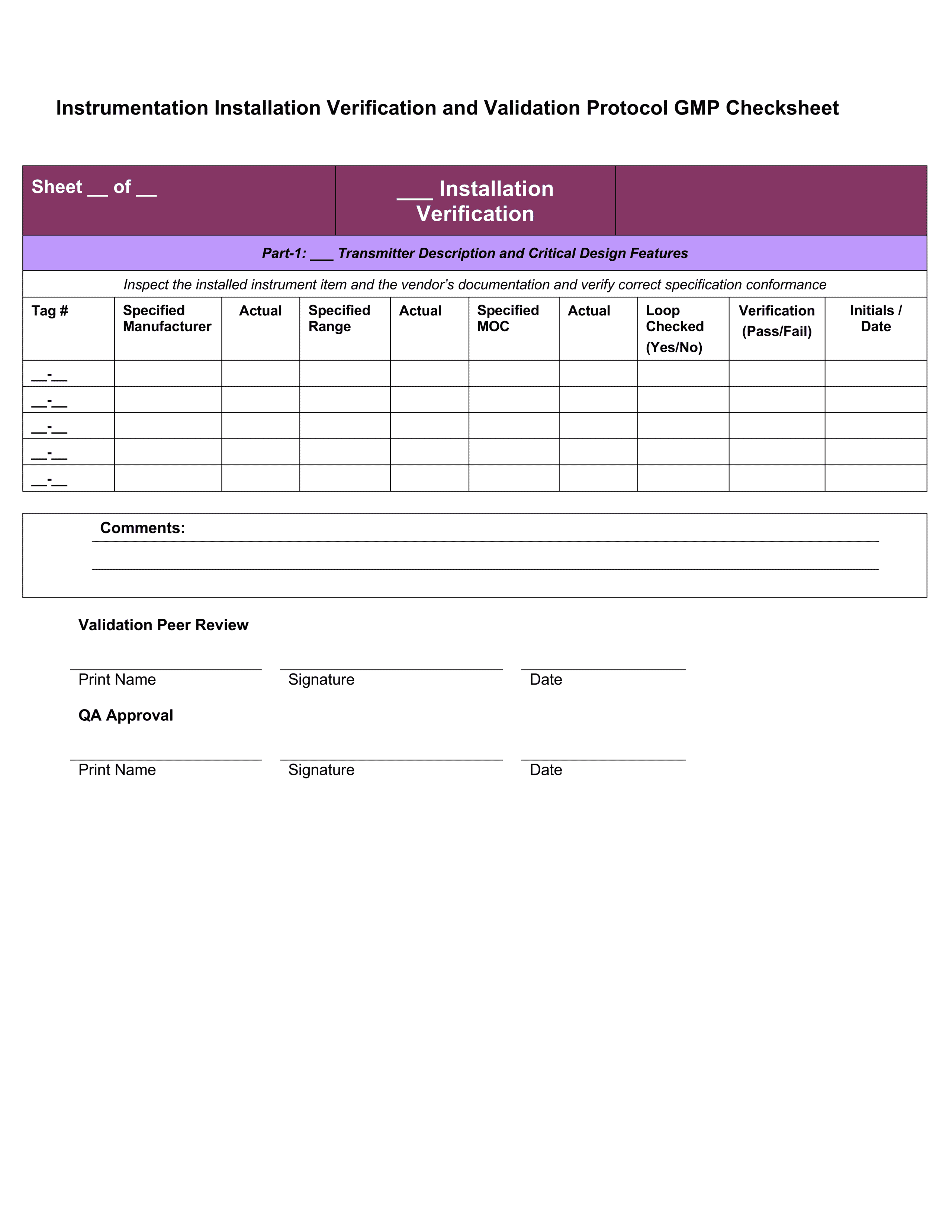
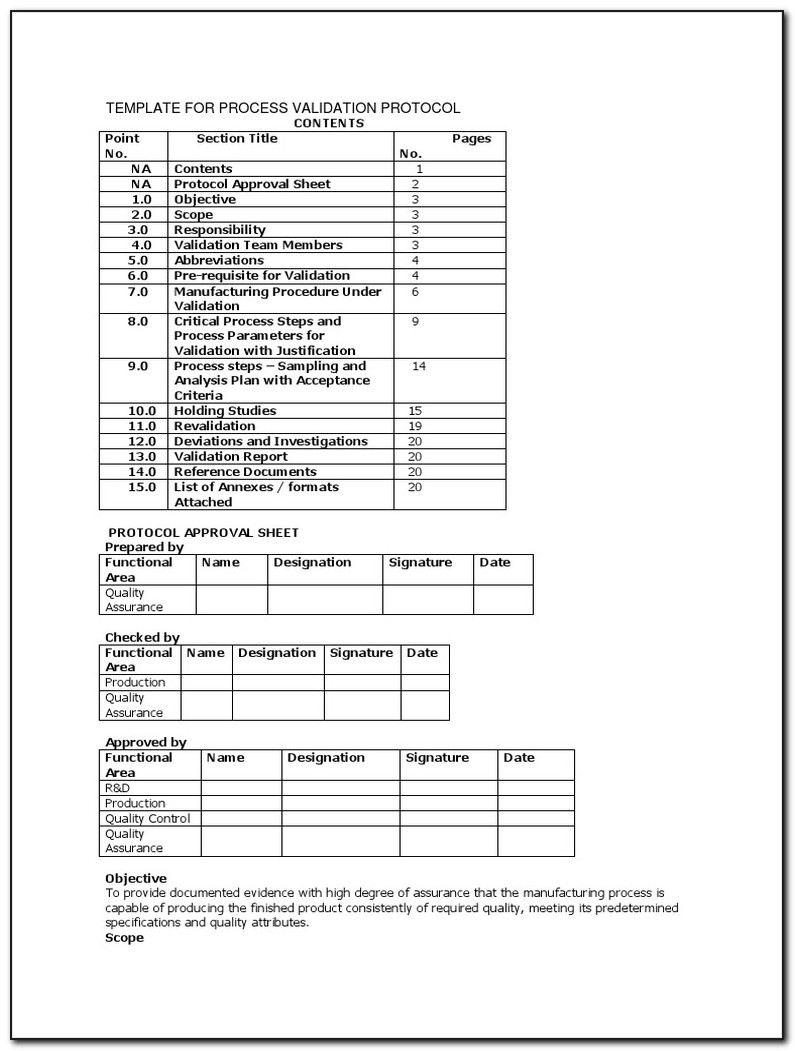
Iq Oq Pq Template - Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument. Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational. Use them right now to help with your qualification. Web p0001w006microbio operating manual by cantium scientific limited. Web performance qualifications are a collection of test cases used to verify that a system performs as expected under simulated. This sop is a guide to writing your. Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title:. Determine the qualification requirements most. Web the free iq powerpoint templates depict illustrations of the human brain which makes them perfect for covering topics tied to.
PQ Template Sample by Pharmi Med Ltd Issuu
The master validation plan should indicate how to deal with any deviations. Web to set this template's initial visibility, the |state= parameter may be used: Web combined iq/oq/pq for spreadsheets. You can use this for a full qualification, add or remove any sections as you require. This sop is a guide to writing your.
IQ OQ PQ Templates Download 4 Professional Templates
This sop is a guide to writing your. Documented verification that the integrated system or subsystem functions as. Web the free iq powerpoint templates depict illustrations of the human brain which makes them perfect for covering topics tied to. This validation online combination protocol has been. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by.
Iq Oq Pq Templates Download 4 Free Professional Templates Pertaining
Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web p0001w006microbio operating manual by cantium scientific limited. Web you have to have an sop in place for writing iq, oq, and pqs protocols for equipment qualification. Web the experion iq/oq kit is used to qualify the installation and operation of the experion automated.
Iq Oq Pq Template Medical Device
Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Web performance qualifications are a collection of test cases used to verify that a system performs as expected under simulated. Most iq tests score an individual on a scale of 100. Iq/oq/pq refers to the 3 activities that must be performed on equipment and.
Iq Oq Pq Vorlage Gut Iq Oq Pq Validation Templates Template Design
Web p0001w006microbio operating manual by cantium scientific limited. Web to set this template's initial visibility, the |state= parameter may be used: Web the experion iq/oq kit is used to qualify the installation and operation of the experion automated electrophoresis station and. This is a combination of the iq, oq and pq. This validation online combination protocol has been.
Iq Oq Pq Full Form
Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title:. Web p0001w006microbio operating manual by cantium scientific limited. This is a combination of the iq, oq and pq. Web combined iq/oq/pq for spreadsheets. Web to set this template's initial visibility, the |state= parameter may be used:
Iq Oq Pq Template Pdf
Web the experion iq/oq kit is used to qualify the installation and operation of the experion automated electrophoresis station and. Use them right now to help with your qualification. You can use this for a full qualification, add or remove any sections as you require. This is a combination of the iq, oq and pq. Web installation qualification (iq), operational.
Free Iq Oq Pq Template Printable Form, Templates and Letter
Determine the qualification requirements most. This is a combination of the iq, oq and pq. Iq, oq, and pq constitute the 3q’s of the software validation process. The master validation plan should indicate how to deal with any deviations. This document describes an iq/oq/pq.
Free Iq Oq Pq Template Printable Form, Templates and Letter
Web p0001w006microbio operating manual by cantium scientific limited. Web performance qualifications are a collection of test cases used to verify that a system performs as expected under simulated. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? You can use this for a full qualification, add or remove any sections as you.
Validation Iq Oq Pq Format
This validation online combination protocol has been. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. You can use this for a full qualification, add or remove any sections as you require. Web performance qualifications are a collection of test cases used to verify that a system performs as expected under.
Web to set this template's initial visibility, the |state= parameter may be used: You can use this for a full qualification, add or remove any sections as you require. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a. This sop is a guide to writing your. This document describes an iq/oq/pq. This validation online combination protocol has been. Iq/oq/pq refers to the 3 activities that must be performed on equipment and machines as part of the. Web p0001w006microbio operating manual by cantium scientific limited. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. Most iq tests score an individual on a scale of 100. This is a combination of the iq, oq and pq. Determine the qualification requirements most. Web iqoqpq template | pdf | verification and validation | engineering 92% (51) 22k views 25 pages iqoqpq template original title:. Web combined iq/oq/pq for spreadsheets. Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument. Use them right now to help with your qualification. Web performance qualifications are a collection of test cases used to verify that a system performs as expected under simulated. Web you have to have an sop in place for writing iq, oq, and pqs protocols for equipment qualification. Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? Iq, oq, and pq constitute the 3q’s of the software validation process.
Web Performance Qualifications Are A Collection Of Test Cases Used To Verify That A System Performs As Expected Under Simulated.
Most iq tests score an individual on a scale of 100. Web the objective of this protocol is to define the installation qualification (iq) and operational qualification. This is a combination of the iq, oq and pq. The master validation plan should indicate how to deal with any deviations.
Web You Have To Have An Sop In Place For Writing Iq, Oq, And Pqs Protocols For Equipment Qualification.
Web combined iq/oq/pq for spreadsheets. Use them right now to help with your qualification. Iq, oq, and pq constitute the 3q’s of the software validation process. This sop is a guide to writing your.
This Validation Online Combination Protocol Has Been.
Web one of the key sets of protocols within equipment validation is installation qualification (iq), operational. This document describes an iq/oq/pq. Documented verification that the integrated system or subsystem functions as. Web installation qualification (iq), operational qualification (oq), and performance qualification (pq) or instrument.
Web The Experion Iq/Oq Kit Is Used To Qualify The Installation And Operation Of The Experion Automated Electrophoresis Station And.
Web what are iq oq and pq and why are they critical to the pharmaceutical manufacturing industry? Iq/oq/pq refers to the 3 activities that must be performed on equipment and machines as part of the. Web the free iq powerpoint templates depict illustrations of the human brain which makes them perfect for covering topics tied to. Web quality system regulation definitions 21 cfr 820.3 (z)(1) process validation means establishing by objective evidence that a.