Clinical Evaluation Report Template - Web clinical investigation application/notification documents: The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. You can download it as. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Web the medical device regulation (mdr) applies from 26 may 2021. Oliver eidel template download this is a free template, provided by openregulatory.
(PDF) Medical device clinical evaluation report (CER) rough template
Oliver eidel template download this is a free template, provided by openregulatory. Web clinical investigation application/notification documents: You can download it as. Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103.
42+ Medical Report Samples Word, PDF, Illustrator
Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Oliver eidel template download this is a free template, provided by openregulatory. Web clinical investigation application/notification documents: The in vitro diagnostics regulation (ivdr) applies from.
Medical Evaluation Report How to create a Medical Evaluation Report
Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Web the medical device regulation (mdr) applies from 26 may 2021. Oliver eidel template download this is a free template, provided by openregulatory. Web.
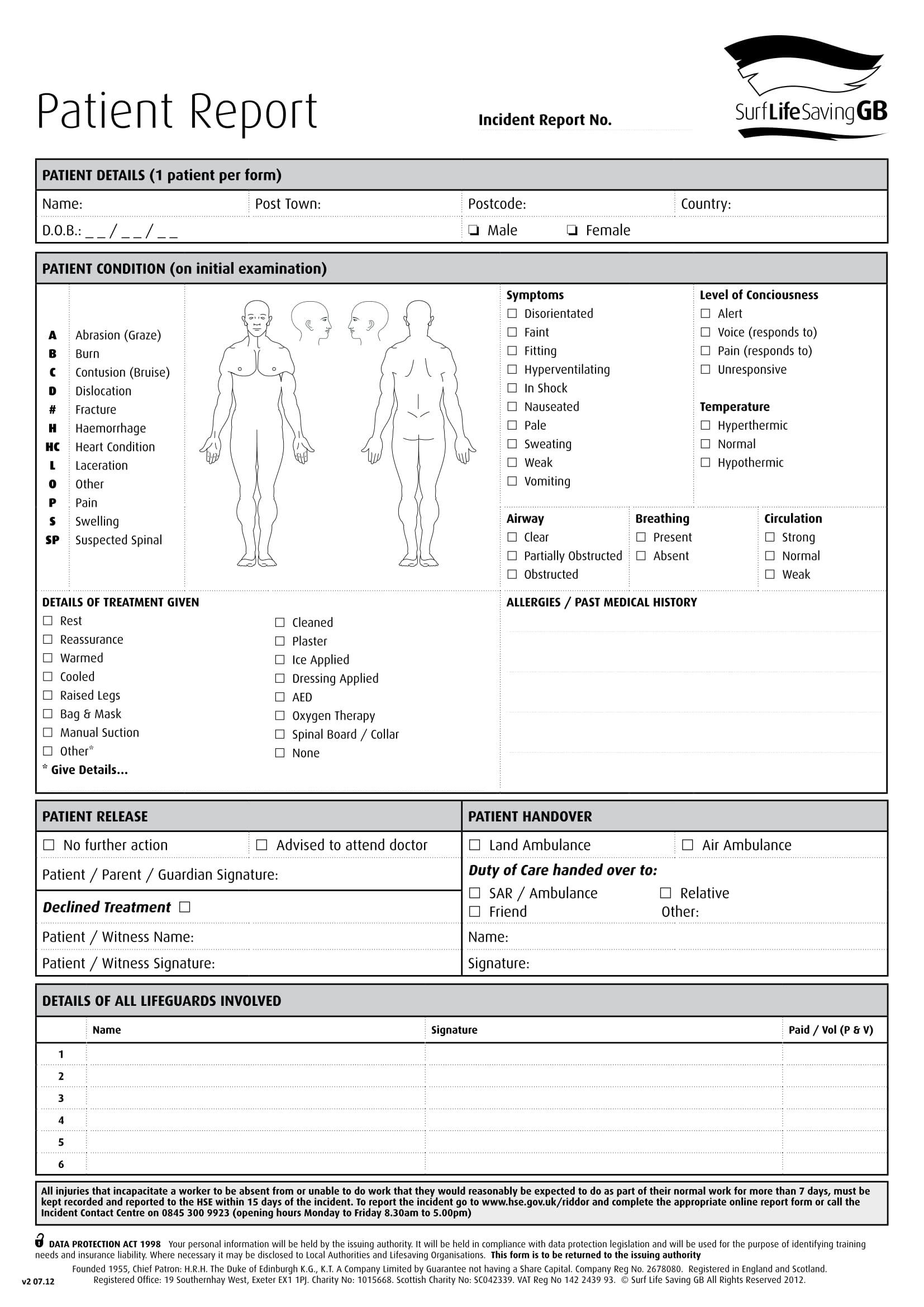
Patient Report Form Template Download Best Template Ideas
Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Oliver eidel template download this is a free template, provided by openregulatory. Web the medical device regulation (mdr) applies from 26 may 2021. Web.
Clinical Evaluation Report Sample
Web the medical device regulation (mdr) applies from 26 may 2021. You can download it as. Web clinical investigation application/notification documents: The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Oliver eidel template download this is a free template, provided by openregulatory.
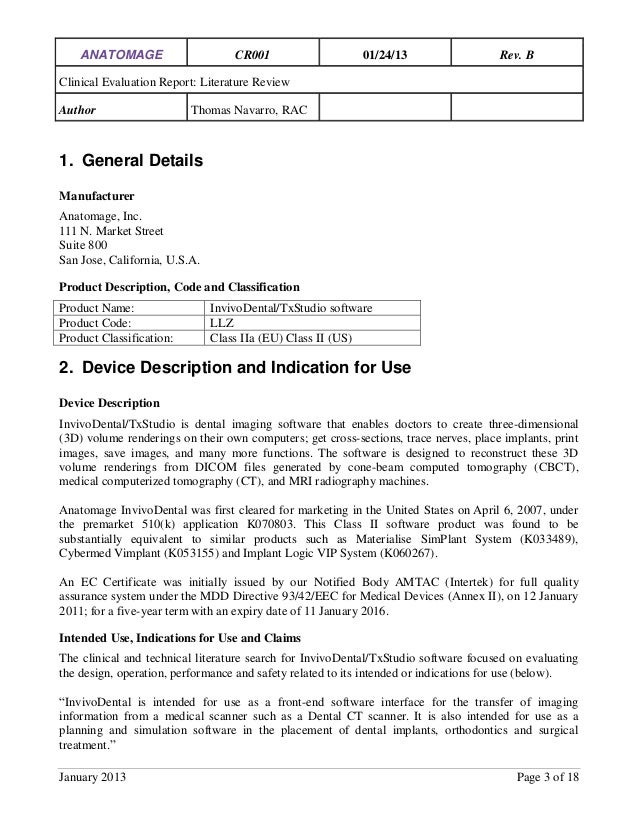
Anatomage_Clinical_Evaluation_ReportTom_Navarro
The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Oliver eidel template download this is a free template, provided by openregulatory. Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical investigation application/notification documents: You can download it as.
Clinical Evaluation Report Template Glendale Community regarding
You can download it as. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Oliver eidel template download this is a free template, provided by openregulatory. Web the medical device regulation (mdr) applies from 26 may 2021. The in vitro diagnostics regulation (ivdr) applies from.
FREE 15+ Sample Evaluation Reports in PDF MS Word Apple Pages
The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Web clinical investigation application/notification documents: You can download it as. Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103.
Clinical Evaluation Plan/Report Fill and Sign Printable Template
Web clinical investigation application/notification documents: Web the medical device regulation (mdr) applies from 26 may 2021. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Oliver eidel template download this is a free template, provided by openregulatory. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg).
Clinical Evaluation Report Template QualityMedDev
Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. You can download it as. Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical investigation application/notification documents:
Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. Oliver eidel template download this is a free template, provided by openregulatory. You can download it as. Web the medical device regulation (mdr) applies from 26 may 2021. Web clinical investigation application/notification documents: The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022.
Web The Medical Device Regulation (Mdr) Applies From 26 May 2021.
The in vitro diagnostics regulation (ivdr) applies from the 22 may 2022. Web clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group (mdcg) established by article 103. You can download it as. Oliver eidel template download this is a free template, provided by openregulatory.